Introducing Latent-X
An Atom-level Frontier Model
for De Novo Protein Binder Design
From Millions of Random Attempts to Reliable Precision Design
Latent Labs introduces Latent-X, our new state-of-the-art frontier AI model for generating lab-functional protein binders at the all-atom level. The model generates designs for distinct therapeutic modalities: macrocycles and mini-binders, achieving breakthrough performance in laboratory validation.
Traditional drug discovery requires screening millions of random molecules—a process where hit rates are typically well below 1% and each experiment takes months and costs thousands of dollars. Latent-X transforms this by using precision AI design that generates reliable binders instantly, solving the geometric puzzle of binding at the all-atom level to directly generate the biochemistry required for high binding affinity and specificity.
The efficiency gains are dramatic. In extensive wet lab experiments, Latent-X achieved what would typically require millions of candidates by testing as little as 30-100 candidates per target—yet achieved strong binding affinities down to the picomolar ranges. The AI model delivered picomolar affinities for mini-binders and up to a 100% hit rate for all tested macrocycles. This outperforms the previous state-of-the-art set by prior models [1, 2, 3].
Full results are available in our technical report. 5 months after its $50M funding announcement, Latent Labs ships its first frontier model. Sign up for early access at platform.latentlabs.com. Commercial and non-commercial use is permitted.
Generating Multiple Therapeutic Binder Modalities with High Success
We validated our generated designs for the two modalities by measuring their binding affinities to benchmark targets including positive controls by prior models. For macrocycles we found binders with single digit micromolar affinities and for mini-binders our strongest binders had picomolar affinities.
Macrocycles
We found Latent-X generated macrocycles to bind to 100% of the three selected benchmark targets. Remarkably, 91-100% of the macrocycles tested for binding were binders. The strongest binders reached single digit micromolar binding affinities and show target specificity in our lab results, a prerequisite for low off-target effects.
Mini-Binders
We found mini-binders for 100% of targets in a benchmark of 5 therapeutically relevant proteins. The best binders achieved picomolar binding affinities, surpassing the strongest reported RFdiffusion and AlphaProteo binders in head-to-head experimental comparison. We show experimentally that the generated mini-binders are highly target specific.
Previewing further binder modalities
Macrocycles and mini-binders are only the start. Latent-X has learnt a lot about protein binding, opening doors to other therapeutic modalities that depend on target-specific binding—nanobodies and antibodies being prime examples. We're excited to explore these opportunities and welcome partnerships to bring these expanded capabilities to new drug applications.
Latent-X’s Distinctive Features
-
Lab-validated binder hits on every tested target
Latent-X generated functional binders on every tested target with 91-100% hit rate for macrocycles and 10-64% hit rate for mini-binders.
-
Stronger binding affinities than prior methods
Across all modalities we found a high incidence of strong binding affinities including pico molar binders that exceeded the binding affinities of designs from other models.
-
Lab validated target specificity
Latent-X directly generates the biochemical bonds to selectively bind user-specified epitopes as shown in lab validation of binding specificity for macrocycles and mini-binders.
-
Model access via no-coding AI platform
Latent-X is available on the Latent Labs Platform, offering the complete lab-validated workflow: target upload, hotspot selection, binder design, and computational ranking.
-
Generalization to multiple therapeutic binder types
Latent-X successfully generates distinct therapeutically relevant binder modalities: macrocycles, protein mini-binders and more to come.
-
Joint protein sequence and structure generation
Latent-X generates all-atom structures, co-sampling sequence and structure, and outperforms prior methods that generate them sequentially.
-
State-of-the-art in silico hit rates
Latent-X outperforms prior methods on in-silico hit rates for targets not seen in its training set requiring fewer samples to arrive at lab-viable numbers of binders.
-
Fast generation speed
Latent-X generates binders over 10x faster than previous methods, reducing generation time to seconds allowing delightfully easy computational experimentation.
Learning the Biochemistry of Binding at the Atom Level
AI models have recently enabled solutions to previously insurmountable technical challenges in biology. With generative models, frontier AI can go beyond predicting structures to creating new sequences and structures of candidate drugs.
Latent-X is a general purpose frontier model that creates binders from scratch for unseen or previously untargeted proteins. This is similar to solving a geometric puzzle where every atom needs precise placement. Latent-X generalizes beyond nature's repertoire by generating all-atom binder structures that obey atomic-level biochemical rules, such as hydrogen bonds and pi-stacking of aromatic rings.
Below we show animations of Latent-X’s iterative generation for macrocycles, mini-binders and nanobodies. The generated binders are shown in an orange to purple gradient, the target is shown in grey, and generated biochemical bonds are displayed in pink.
Latent Labs Platform Available Now
Latent-X is available on the Latent Labs Platform, enabling non-coding users and users without access to AI infrastructure to reliably generate successful de novo binders. The platform includes a free tier and replicates our published lab-validated AI workflows: scientists can upload targets, specify epitopes, design binders, and rank designs by computational success metrics for lab testing. Currently supporting macrocycles and mini-binders with more features coming, the platform provides structure visualization, predicted structure overlays, and computational metric rankings.
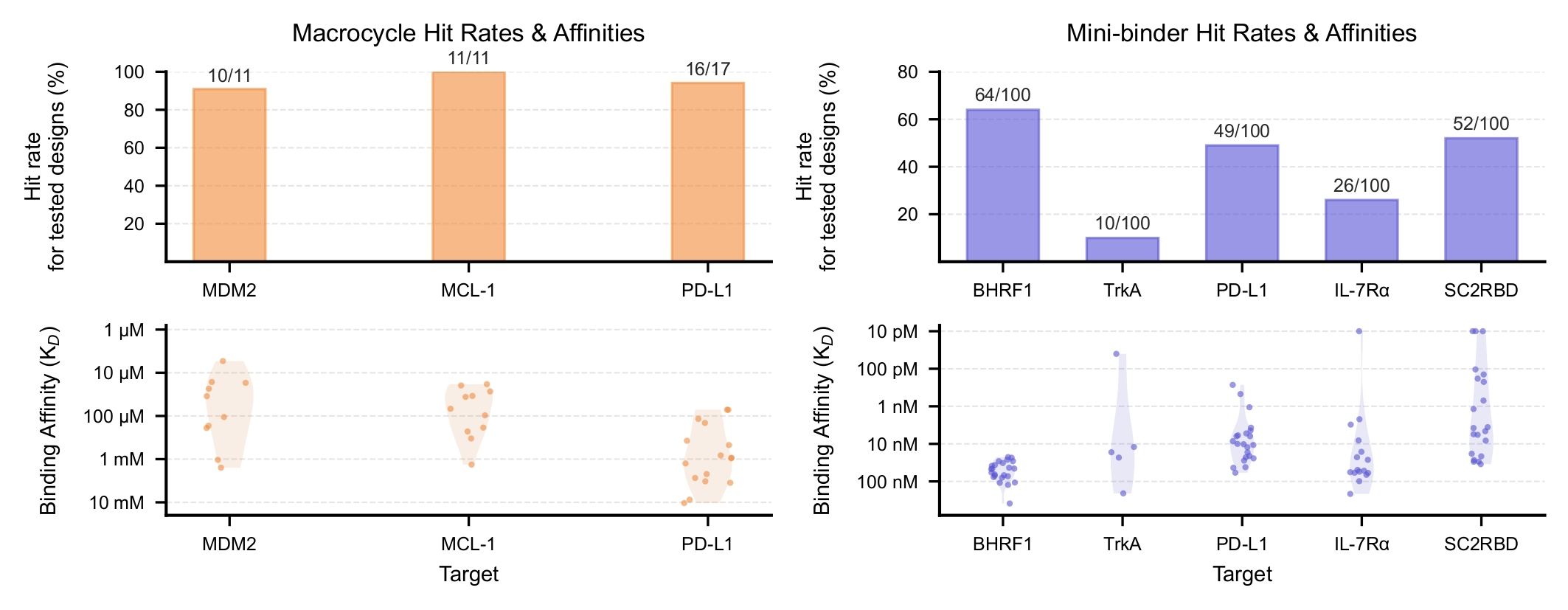
Extensive Lab Validation with High Success Rates and Affinities
The charts below detail our comprehensive validation across 7 therapeutic targets, testing fewer than 30 macrocycles and 100 mini-binders per target. The bar charts show hit rates of 91-100% for macrocycles across three targets and 10-64% for mini-binders across five targets. The binding affinity distributions reveal macrocycles performing consistently in the low micromolar range, while mini-binders achieved picomolar binding strength across all targets tested.
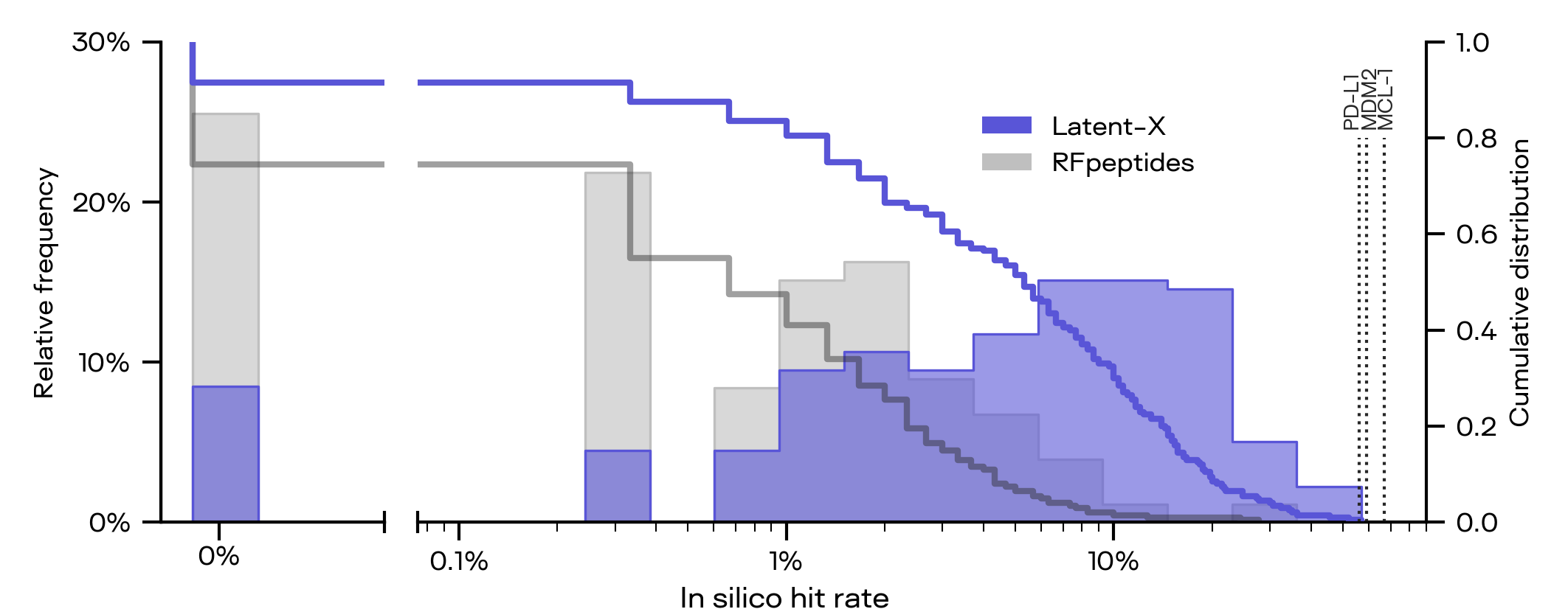
State-of-the-Art In Silico Hit Rates
Latent-X achieves significantly higher computational hit rates on a large evaluation set of held-out binder targets, strongly outperforming prior methods, see the strong computational hit rates achieved by Latent-X for macrocycles below. With generation speeds over 10x faster, improved further in batched mode, Latent-X efficiently samples lab-viable quantities of high-confidence designs that pass computational success criteria.
Fast delivery of our wide access platform
The Latent Labs Platform is open for sign-up today at platform.latentlabs.com. The platform includes a free tier for all users with user credits renewing daily.
We want to quickly empower a broad community of scientists with our cutting edge technology so we have chosen to launch with multiple access mechanisms including free tiers. This gets cutting-edge AI into the hands of both bigger and smaller companies, without the need to set up AI teams or AI infrastructure.
As Latent Labs CEO and founder Simon Kohl notes: "We envision a future where effective therapeutics can be designed entirely in a computer, much like how space missions or semiconductors are designed today. Our platform empowers scientists with lab-validated protein binder design at their fingertips, whether they're experts or new to AI-powered drug design. This is the first step on our mission toward making biology programmable in order to make drug design instantaneous."
In February 2025 Latent Labs announced its $50M funding round co-lead by Radical Ventures and Sofinnova Partners. The team consists of former AlphaFold 2 co-developers, ex-DeepMind team leads, and brings rich experience from Microsoft, Apple, Stability AI, Exscientia, Mammoth Bio, Altos Labs and Zymergen.