Latent-X2
Drug-like antibodies with low immunogenicity in human panels
Designing Drug-Like Molecules from the Start
Latent Labs introduces Latent-X2, our frontier AI model for designing high-affinity antibodies with drug-like properties, alongside macrocyclic peptides. Five months after launching Latent-X1, we've taken the next step: molecules that don't just bind, but can clear developability and immunogenicity hurdles.
Traditional drug discovery faces a bottleneck not in screening, but in development. Hits rarely possess the properties needed for clinical success, and efforts to address shortcomings frequently improve one property at the expense of another. This requires long development timelines, and suboptimal starting points risk costly failure downstream in clinical programs.
Latent-X2 changes this. The model jointly generates all-atom structures and sequences conditioned on multi-modal prompts—target structure, epitope specification, and optional antibody framework. Testing only 4 to 24 designs per target, we achieve high-affinity de novo binders across antibodies and macrocyclic peptides, with drug-like properties for antibodies from the first generation.
Full results are available in our technical report. Apply for early access at platform.latentlabs.com.
Zero-Shot Antibody and Peptide Design
We validated Latent-X2 across targets selected for diversity and difficulty. The model generates high-affinity binders across multiple modalities.
Nanobodies (VHHs)
Nanobodies are single-domain antibodies derived from camelids, valued for their small size and ability to access epitopes inaccessible to conventional antibodies.In our lab experiments, Latent-X2 generates VHH binders with nanomolar affinities.
Single-Chain Variable Fragments (scFvs)
scFvs combine the variable regions of antibody heavy and light chains in a single polypeptide, offering flexibility for therapeutic and diagnostic applications.In our lab experiments, Latent-X2 generates scFv binders with down to low picomolar binding affinities.
Macrocyclic Peptides
Macrocyclic peptides are small cyclized proteins that are a promising new drug class for their potential oral bioavailability.Latent-X2 generates macrocyclic peptides that bind K-Ras—long considered undruggable—matching or exceeding the affinity of hits from trillion-scale mRNA display screens while testing 11 orders of magnitude fewer sequences.
Drug-Like by Default
Our designed antibodies exhibit developability profiles—expression yield, biophysical characteristics, polyreactivity—comparable to approved therapeutics in head-to-head comparison. These properties arise from the first generation, without optimization, filtering, or selection
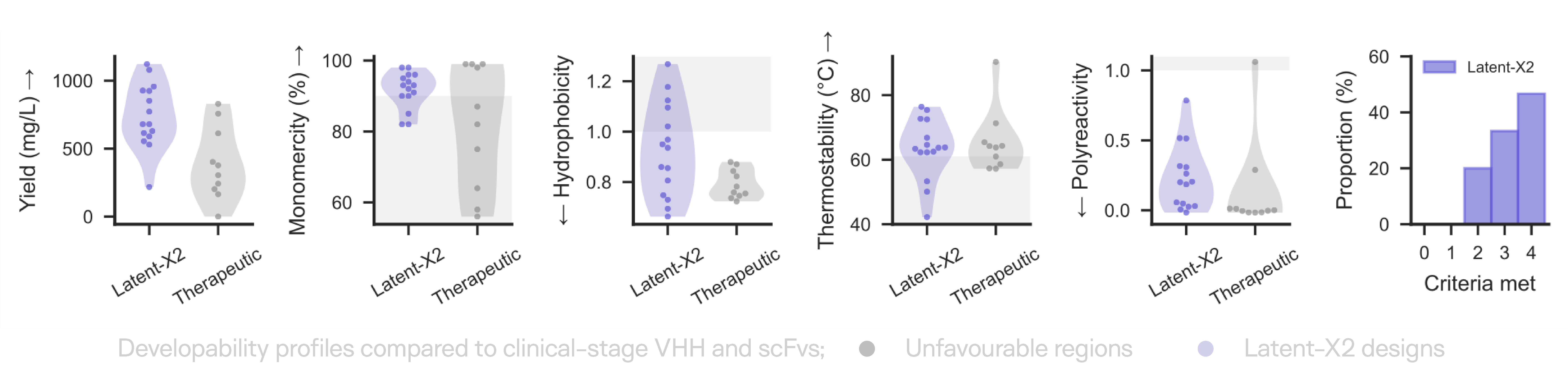
This extends to immunogenicity. In the first such assessment of any AI-generated antibody, de novo VHH binders targeting TNFL9 were evaluated across a ten-donor human panel in ex vivo T-cell activation and cytokine release assays, confirming both potent target engagement and low immunogenicity. This is the first time an AI-generated antibody has been shown to clear immunogenicity assessment—a critical milestone for clinical translation.
While animal studies and clinical trials remain ahead, these results demonstrate that AI-designed molecules can now clear drug development hurdles that previously required years of optimization.
Latent Labs Platform
The Latent Labs platform makes it easy to generate antibody and peptide candidates for a particular target. It produces high-affinity binders across VHH, scFv, and macrocyclic peptide formats—with antibodies approaching drug-like quality from the first generation.
The platform provides scientist-friendly workflows to access Latent-X2. Customers can access the platform in a web browser or integrate their systems with the Latent Labs API.
Latent-X2 builds on the success of Latent-X1, released just five months ago. Since then, Latent-X1 has been adopted by industry and academic groups worldwide, who value its performance and no-code interface for real lab applications.
Latent-X2's Distinctive Features
-
Drug-like from first generation
Developability and low ex vivo immunogenicity arise without optimization, filtering, or selection.
-
First low immunogenicity demonstration
De novo antibody designs confirmed across human donor panels in ex vivo assays.
-
Broad modality coverage
VHH, scFv, and macrocyclic peptide formats from a single model.
-
Hits on difficult targets
Including historically challenging and undruggable targets, with affinities matching trillion-scale screens.
-
Lab-compatible efficiency
Just 4 to 24 designs per target, versus millions or trillions in conventional screening.
-
Precise model steering
Condition on target structure, epitope, and antibody framework of choice.
-
All-atom generation
Joint sequence and structure generation modeling non-covalent interactions in a single step.
How Latent-X2 Works
Latent-X2 is an all-atom generative model that jointly generates sequences and structures while modeling the bound complex. By directly modeling non-covalent interactions, the model respects the biochemistry of binding and function at the atomic level.
The model is conditioned on multi-modal prompts: the target structure defines what to bind, the epitope specification defines where, and an optional antibody framework allows following a preferred scaffold. This enables precise control over the design process across different biological modalities.
Discussion
To allow for reproduction we publish the sequence and structure of a lab-validated antibody designs for each target on the Latent Labs Platform, accessible without sign-in.
All results presented here are preclinical, with animal studies and clinical trials remaining ahead. Immunogenicity assessment was conducted ex vivo using human donor panels—a state-of-the-art proxy for clinical immunogenicity risk, but not a substitute for in vivo validation. Extending this validation across additional targets and modalities is a natural next step.
Access
Latent-X2 is available on the Latent Labs Platform. Apply for early access at partnerships@latentlabs.com.
"Semiconductors, satellites and aircraft once required repeated build-test cycles, consuming years and billions of dollars. Today they're designed computationally before anything is fabricated. With Latent-X2, drug discovery can move towards that same step change—designing the right molecule from the start," said Simon Kohl, CEO and founder of Latent Labs.
"The pharmaceutical industry has spent decades optimizing around the limitations of iterative lab work. Latent Labs is doing something different—building the capability to design molecules that work from first principles. That shift, if it holds, changes the entire logic of drug discovery," said Stefan Oschmann, former CEO of Merck KGaA and newly appointed Latent Labs strategic advisor.
Latent Labs announced its $50M funding round ten months ago, co-led by Radical Ventures and Sofinnova Partners, with participation by Anthropic's CEO Dario Amodei, Eleven Labs' CEO Mati Staniszewski, and Google's Chief Scientist Jeff Dean. The team includes former AlphaFold 2 co-developers and ex-DeepMind team leads, with experience from Microsoft, Apple, Exscientia, Mammoth Bio, Altos Labs, and Zymergen.